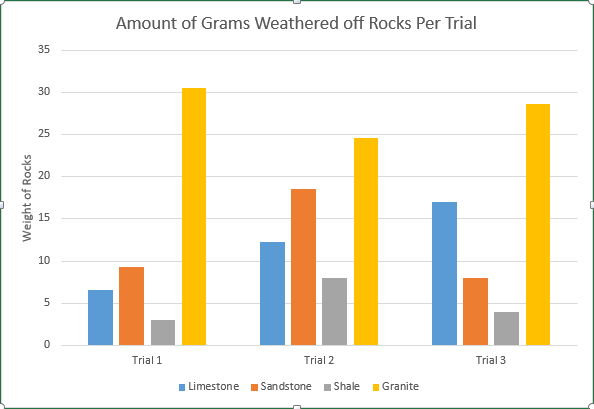
Rocks come in different shapes and sizes, but no one rock is “stable or immune to weathering. Weathering is the alteration of rocks from their exposure to the agents of air, water, and organic fluids” (Burns, 2003). Although all rocks are affected by weathering, each rock takes a certain amount of chemical or mechanical exposure to define the rate at which it weathers. By investigating the question ‘Does limestone chemically and mechanically weather faster than granite, sandstone, and shale?’it will help support the opinion that the rate of which limestone weathers is more rapid than that of granite, sandstone, and shale from abrasion and the altering of pH levels of diluted acid that will be used to show weathering.
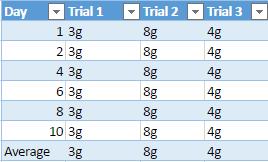
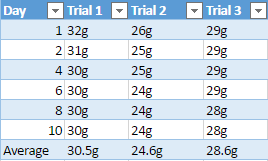
Chemical weathering is when a rock is slowly weathered away from chemical reactions that happen from water and a combination of different gases. Granite one of the rocks being used in the experiment is known to weather slower than most rocks, for example a group of scientist from Queen’s University in Belfast have investigated the weathering of a lighthouse, which uses granite as its interior structure. The granite used in the lighthouse, which was built over a century ago is starting to show signs of harsh weathering from “long-term exposure to aggressive maritime conditions”, which are causing serious problems (Warke,2002). Although the granite is now starting to show some signs of decay, it took over a hundred years and exposure to aggressive water to do so. This greatly shows that out of the three rock samples being tested against limestone, that granite will most likely show less weathering through the duration of the experiment.
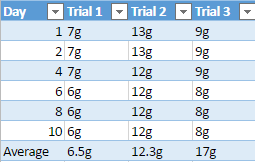
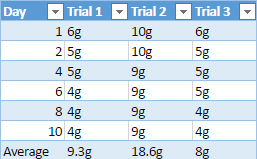
Mechanical weathering is when rocks break down into smaller pieces, usually caused by change in temperature or natural forces such as tree roots that grow around rocks. The most common example of mechanical weathering is the “repeated freezing and thawing of water in rock crevices and soil material” (Fraser, 1959). We see limestone used in very important things, such as cement which is used to make buildings and sidewalks. We often see that sidewalks are cracked which is caused by heating and cooling that eventually break down the cement. Since limestone is very soluble from water and “the more acidic the water is the more [the] limestone will react” causing the limestone to eventually start to weather and erode away (Senese, 1997).
During the experiment to support the idea that limestone will weather away faster, the pH of diluted acid being used on all four rocks will be alternated every day to show the effect of acidic rain, which causes a lot of weathering and erosion for rocks. The mineral composition of limestone will also play a heavy factor in this experiment because limestone is a sedimentary rock that is mostly composed of calcite and aragonite, it is also composed of skeletal fragments of sea organisms. Limestones mineral composition is one of the main reasons why it is hypothesized it will weather faster than the other three rock samples.